Featured cases
UNiDTM Adaptive Spine Intelligence Case Library
Check back weekly for additional content featuring UNiDTM cases!
September 2022
Complex UNiDTM ASI T10-Pelvis correction
Patient description: 70 year-old female with severe low back pain and fatigue exacerbated by standing/walking, improved with sitting/supine position. Also with left L3/4/5 radiculopathy, neurogenic claudication. Right sided hip flexor and quadricep weakness.
Patient diagnosis: Multilevel lumbar spondylosis, spinal stenosis, L3-4 and L4-5 degenerative spondylolisthesis, sagittal plane malalignment with thoracolumbar deformity. Patient was also noted to have severe sagittal plane malalignment with:
Cranial SVA-H = 85mm
SVA = 90mm
TPA = 26°
L1PA = 26°
Pelvic Tilt = 22°
PI = 60° & SS = 38°, suggestive of Roussouly type 3 or 4 morphology
Interestingly, her PI-LL mismatch was only 8°. The distribution of her lumbar lordosis from L4-S1 was quite low (5°) while from L1-4 was supraphysiologic (45°). She essentially had a 30°+ lordotic deficit from L4-S1 and ~25° lordotic surplus from L1-L4. Due to the loss of lordosis and spondylolisthesis at L4-S1, the patient was engaging compensatory mechanisms including pelvic retroversion and upper lumbar hyperlordosis.

Presented by:
David Ou-Yang, M.D.
University of Colorado
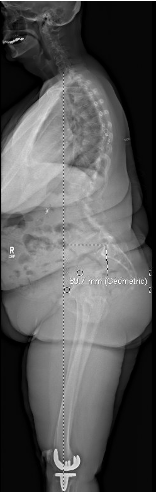
Sagittal pre-operative X-ray
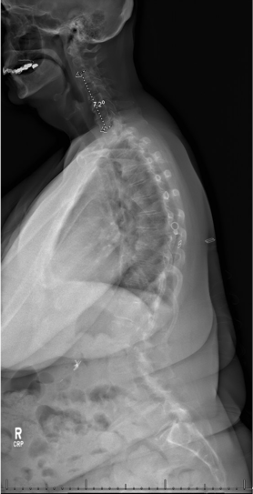
Sagittal pre-operative X-ray
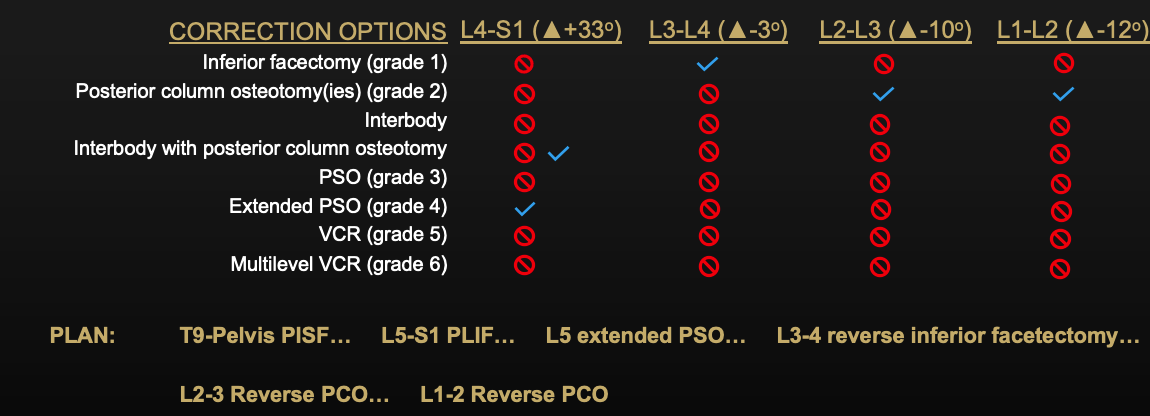
Surgical Plan
Case description:
- T10-Pelvis posterior instrumented fusion with L5-S1 PLIF to achieve 10° of correction across the lowest segment
- Expandable PLIF cages allowed for tailored lordotic correction
- 25° pedicle subtraction osteotomy at L5 extending into the L4-5 disc space was performed to facilitate spondylolisthesis reduction (large disc osteophyte complex was a barrier to this without extending the osteotomy into the disc space)
- Smith Peterson osteotomies were performed at L1-2, L2-3, L3-4 to allow for correction of the upper lumbar hyperlordosis from L1-4
- Once the osteotomies were performed to adequately mobilize the spine, a pre-contoured patient-specific UNiDTM rod was utilized to facilitate lordotic correction (both increase in lordosis from L4-S1 and decrease in lordosis from L1-L4).
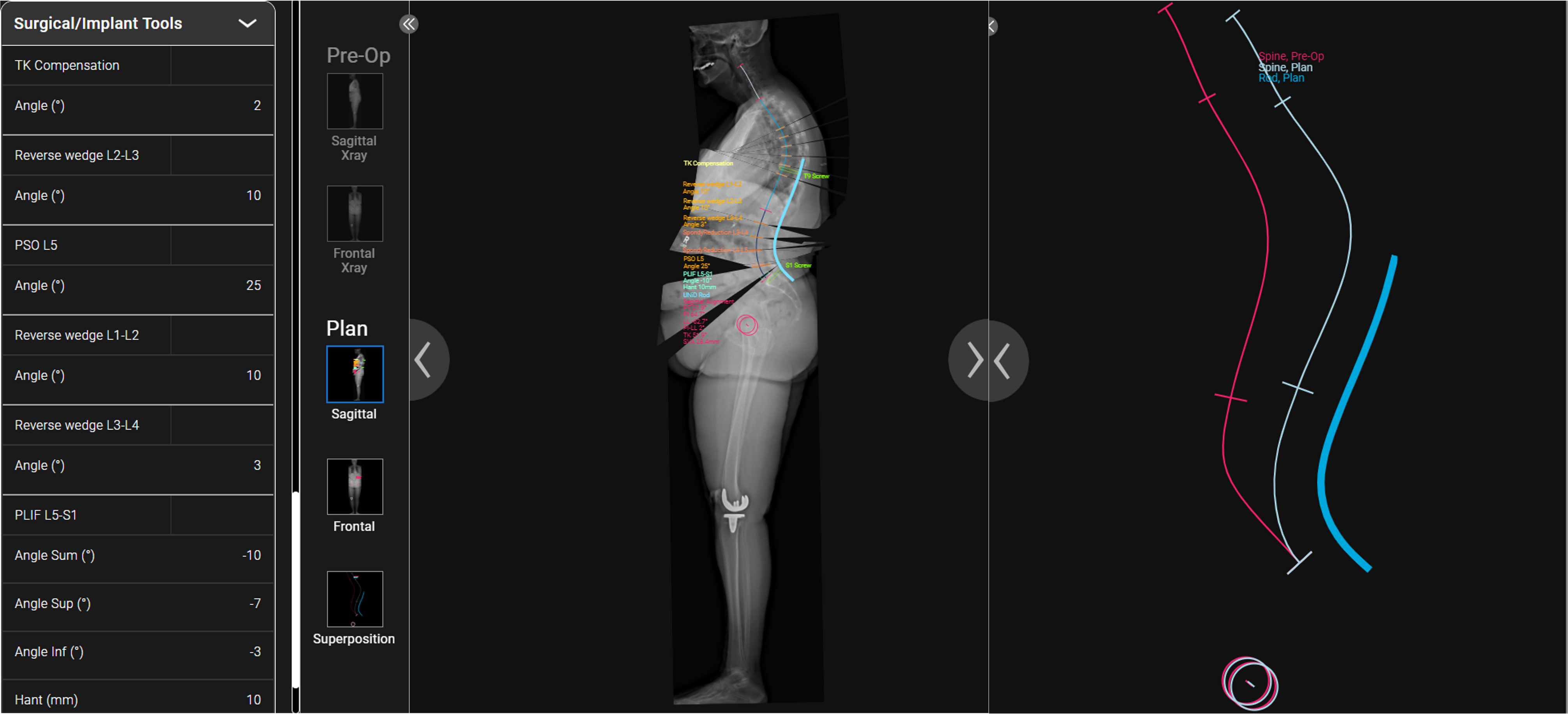
UNiDTM Hub Plan
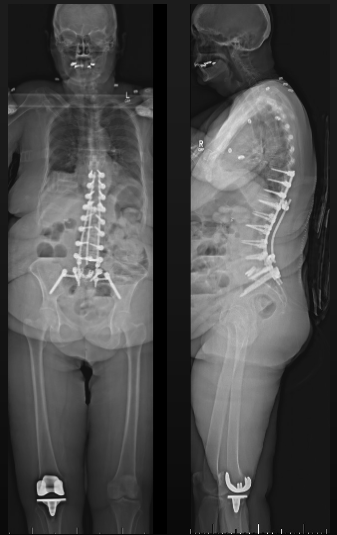
Post-Op X-Ray
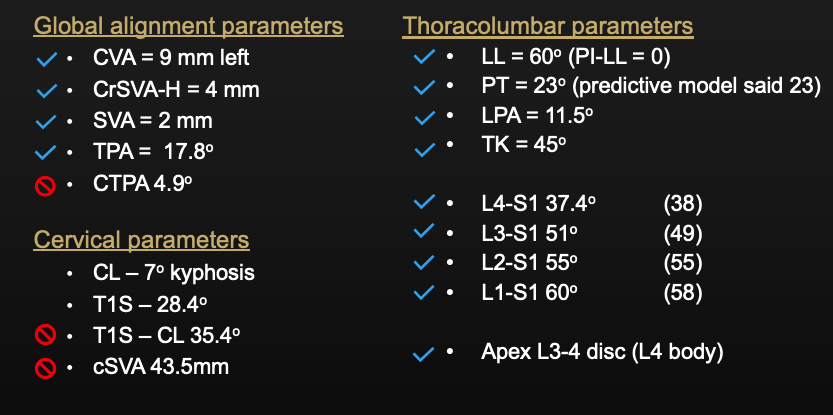
Radiographic outcomes:
- Cranial SVA-H improved from 85mm to 4mm
- SVA improved from 90mm to 2mm
- TPA improved from 26° to 18°
- PT remained at 23 (as predictive model suggested)
- L1PA improved from 26° to 11°
- Lordosis from L4-S1 improved from 5° to 38°
- Lordosis from L1-4 improve from 45° to 22°
All patient reported outcome metrics improved & met minimally clinical important difference.
- PROMIS Pain interference score improved from 72 preop to 54 at 6 months post op.
- PROMIS Physical function score improved from 27 preop to 45 at 6 months post op.
- Oswestry Disability Index improved from 51 preop to 32 6 months post op.
Post-Op Parameters
Dr. David Ou-Yang is a paid consultant for Medtronic.
UNiD™ Spine Analyzer
The UNiD™ Spine Analyzer is intended for assisting healthcare professionals in viewing and measuring images as well as planning orthopedic surgeries. The device allows surgeons and service providers to perform generic, as well as spine related measurements on images, and to plan surgical procedures. The device also includes tools for measuring anatomical components for placement of surgical implants. Clinical judgment and experience are required to properly use the software.
The PASS LP® spinal systems
he PASS LP® spinal systems include a pedicle system intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion in the treatment of the following acute and chronic instabilities or deformities of thoracic, lumbar, and sacral spine:
- Fractures.
- Dislocation.
- Failed previous fusion (pseudarthrosis).
- Spinal stenosis.
- Degenerative spondylolisthesis with objective evidence of neurological impairment.
- Spinal deformations such as scoliosis or kyphosis.
- Loss of stability due to tumors.
The PASS LP® spinal systems are also indicated for pedicle screw fixation for the treatment of severe spondylolisthesis (Grades 3 and 4) of the L5–S1 vertebra in skeletally mature patients receiving fusion by autogenous bone graft having implants attached to the lumbar and sacral spine (L3 to sacrum) with removal of the implants after the attainment of a solid fusion. The PASS LP® also include hooks and rods and sacral/iliac screws indicated for degenerative disc disease (ddd) defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies, spondylolisthesis, trauma (i.e., fracture or dislocation), spinal stenosis, deformities or curvatures (i.e., scoliosis, kyphosis, and/or lordosis), tumor, pseudarthrosis and failed previous fusion. Except for rod plates, when used for posterior non-cervical pedicle screw fixation in pediatric patients, the PASS LP® spinal system implants are indicated as an adjunct to fusion to treat adolescent idiopathic scoliosis. The PASS LP® spinal system is intended to be used with allograft and/or autograft. Pediatric pedicle screw fixation is limited to a posterior approach.
WARNING:
The safety and effectiveness of this device has not been established for use as part of a growing rod construct. This device is only intended to be used when definitive fusion is being performed at all instrumented levels.
RISKS:
In addition to the risks associated with surgery of the spine without instrumentation, a number of possible undesirable effects may occur with instrumented surgery (including but not limited to):
Detachment, deformation, mobilization, slipping, breakage of one or all of the components.
Pain due to the surgery, the fracture, deformation and or migration of an implant.
Fracture of the pedicle during insertion of a pedicular screw.
Postoperative loss of correction and/or reduction of the spine, partial or total loss of the corrections achieved.
CD Horizon™ Spinal System
The CD Horizon™ Spinal System with or without Sextant™ instrumentation is intended for posterior, non-cervical fixation as an adjunct to fusion for the following indications: degenerative disc disease (DDD - defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), spondylolisthesis, trauma (i.e. fracture or dislocation), spinal stenosis, curvatures (i.e. scoliosis, kyphosis, or lordosis), tumor, pseudarthrosis, and/or failed previous fusion.
Except for hooks, when used as an anterolateral thoracic/lumbar system, the CD Horizon™ Spinal System titanium, cobalt chrome, and stainless steel implants may also be used for the same indications as an adjunct to fusion.
With the exception of DDD, CD Horizon™ Legacy™ 3.5mm rods and associated components may be used for indications in skeletally mature patients as an adjunct to fusion. The 3.5mm rods may be used for the specific pediatric indications noted. When used for posterior non-cervical pedicle screw fixation in pediatric patients, CD Horizon™ Spinal System titanium, cobalt chrome, and stainless steel implants are indicated as an adjunct to fusion to treat progressive spinal deformities (i.e. scoliosis, kyphosis, or lordosis) including idiopathic scoliosis, neuromuscular scoliosis, and congenital scoliosis. Additionally, the CD Horizon™ Spinal System is intended to treat pediatric patients diagnosed with the following conditions: spondylolisthesis/ spondylolysis, fracture caused by tumor and/or trauma, pseudarthrosis, and/or failed previous fusion. These devices are to be used with autograft and/or allograft. Pediatric pedicle screw fixation is limited to a posterior approach.
The CD Horizon™ PEEK rods are intended to provide posterior supplemental fixation when used with an interbody fusion cage for patients diagnosed with DDD. These DDD patients may also have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level. This device is intended for 1-2 level use in the lumbosacral spine (L2 – S1) in skeletally mature patients. Devices are intended for use with an interbody fusion cage at the instrumented level and is not intended for stand-alone use.
The CD Horizon™ Spire™ plate is a posterior, single-level, non-pedicle supplemental fixation device intended for use in the non-cervical spine (T1-S1) as an adjunct to fusion in skeletally mature patients. It is intended for plate fixation/attachment to spinous processes for the purpose of achieving supplemental fixation in the following conditions: DDD, spondylolisthesis, trauma, and/or tumor.
To achieve additional levels of fixation, CD Horizon™ Spinal System rods may be connected to the Vertex™ Reconstruction System with the Vertex™ rod connector. Refer to the Vertex™ Reconstruction System package insert for a list of Vertex™ indications.
©2022 Medtronic. All rights reserved. Medtronic, Medtronic logo and Further, Together are trademarks of Medtronic. All other brands are trademarks of a Medtronic company.
UC202305359EN CST
