
Featured cases
UNiDTM Adaptive Spine Intelligence Case Library
Check back regularly for additional content featuring UNiDTM cases!
June 2023
L4-S1 fusion featuring UNiDTM ASI patient-specific rods, Mazor XTM System, CD HorizonTM SoleraTM VoyagerTM with AnteralignTM TL and LS
Patient description: 59-year-old male with a BMI of 38 who presents with back and bilateral leg pain.
Patient diagnosis: L5 - S1 spondylolisthesis with L4-5 spondylosis

Presented by:
Martin H. Pham, M.D.
UC San Diego
Paid Consultant for Medtronic
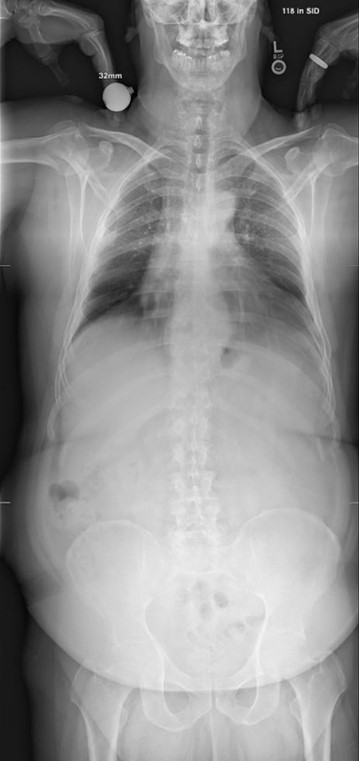
Sagittal Pre-operative Imaging
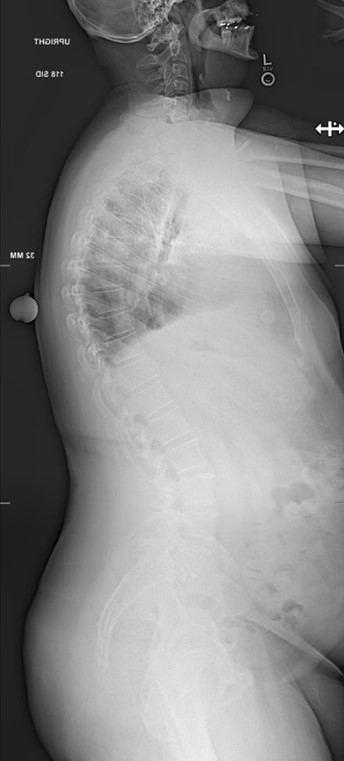
Lateral Pre-operative Imaging
Case description: Patient underwent a minimally invasive single position L4-S1 oblique lumbar interbody fusion with posterior spinal fixation. The UNiDTM Hub predictive analysis powered by UNiDTM Spine Analyzer was used to ensure that the implants planned would keep him in good alignment. The Mazor X Robotics system was used for single position surgery to allow for placement of posterior Voyager screws in the lateral decubitus position, followed by placement of titanium Anteralign LS (L5-S1) and Anteralign TL (L4-5) titanium cages for the OLIF. An O-armTM Surgical Imaging System confirmation spin was then performed to ensure that everything was appropriately placed prior to leaving the OR.
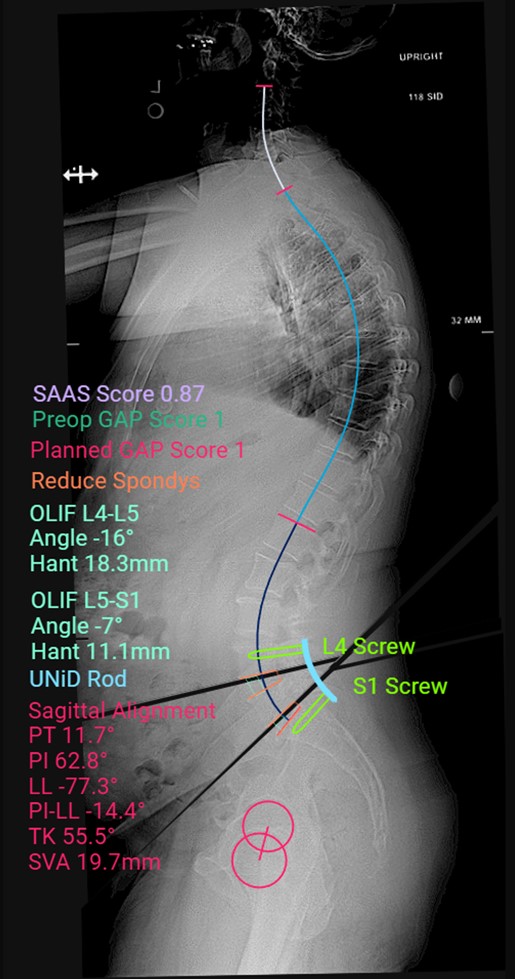
UNiDTM HUB Plan
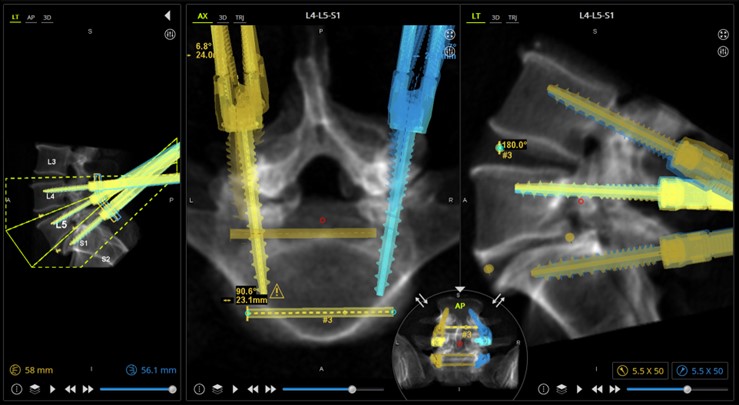
MazorTM Screw Plan
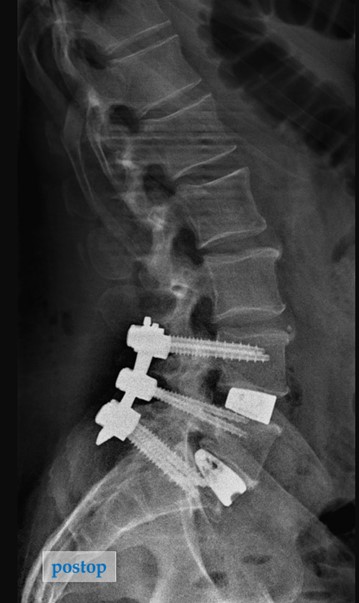
Lateral Post-operative Imaging
Outcome: At the 3-month follow-up, he has had resolution of his back and leg pain and is back to full activities including traveling and hiking.
Dr. Martin H. Pham is a paid consultant for Medtronic.
UNiD™ Spine Analyzer
The UNiD™ Spine Analyzer is intended for assisting healthcare professionals in viewing and measuring images as well as planning orthopedic surgeries. The device allows surgeons and service providers to perform generic, as well as spine related measurements on images, and to plan surgical procedures. The device also includes tools for measuring anatomical components for placement of surgical implants. Clinical judgment and experience are required to properly use the software.
The PASS LPTM spinal systems
The PASS LPTM spinal systems include a pedicle system intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion in the treatment of the following acute and chronic instabilities or deformities of thoracic, lumbar, and sacral spine:
Fractures.
Dislocation.
Failed previous fusion (pseudarthrosis).
Spinal stenosis.
Degenerative spondylolisthesis with objective evidence of neurological impairment.
Spinal deformations such as scoliosis or kyphosis.
Loss of stability due to tumors.
The PASS LPTM spinal systems are also indicated for pedicle screw fixation for the treatment of severe spondylolisthesis (Grades 3 and 4) of the L5–S1 vertebra in skeletally mature patients receiving fusion by autogenous bone graft having implants attached to the lumbar and sacral spine (L3 to sacrum) with removal of the implants after the attainment of a solid fusion. The PASS LPTM also include hooks and rods and sacral/iliac screws indicated for degenerative disc disease (ddd) defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies, spondylolisthesis, trauma (i.e., fracture or dislocation), spinal stenosis, deformities or curvatures (i.e., scoliosis, kyphosis, and/or lordosis), tumor, pseudarthrosis and failed previous fusion. Except for rod plates, when used for posterior non-cervical pedicle screw fixation in pediatric patients, the PASS LPTM spinal system implants are indicated as an adjunct to fusion to treat adolescent idiopathic scoliosis. The PASS LPTM spinal system is intended to be used with allograft and/or autograft. Pediatric pedicle screw fixation is limited to a posterior approach.
WARNING:
The safety and effectiveness of this device has not been established for use as part of a growing rod construct. This device is only intended to be used when definitive fusion is being performed at all instrumented levels.
RISKS:
In addition to the risks associated with surgery of the spine without instrumentation, a number of possible undesirable effects may occur with instrumented surgery (including but not limited to):
Detachment, deformation, mobilization, slipping, breakage of one or all of the components.
Pain due to the surgery, the fracture, deformation and or migration of an implant.
Fracture of the pedicle during insertion of a pedicular screw.
Postoperative loss of correction and/or reduction of the spine, partial or total loss of the corrections achieved.
Mazor XTM System
The Mazor XTM system is indicated for precise positioning of surgical instruments or spinal implants during general spinal surgery. It may be used in either open or minimally invasive or percutaneous procedures. Spinal implants are limited for use in certain disease states and spinal procedures.
The Stealth-Midas™ system and The Midas Rex™ Attachments and Dissecting Tools for Mazor™ system are indicated for the incision/cutting, drilling, burring, and removal of hard tissue and bone in open and minimally invasive spine procedures.
Mazor™ 3-D imaging capabilities provide a processing and conversion of 2-D fluoroscopic projections from standard C-Arms into volumetric 3-D image. It is intended to be used whenever the clinician and/or patient benefits from generated 3-D imaging of high contrast objects.
The Mazor™ navigation tracks the position of instruments, during spinal surgery, in relation to the surgical anatomy and identifies this position on diagnostic or intraoperative images of a patient.
CD Horizon™ Spinal System
The CD Horizon™ Spinal System with or without Sextant™ instrumentation is intended for posterior, non-cervical fixation as an adjunct to fusion for the following indications: degenerative disc disease (DDD - defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), spondylolisthesis, trauma (i.e. fracture or dislocation), spinal stenosis, curvatures (i.e. scoliosis, kyphosis, or lordosis), tumor, pseudarthrosis, and/or failed previous fusion.
Except for hooks, when used as an anterolateral thoracic/lumbar system, the CD Horizon™ Spinal System titanium, cobalt chrome, and stainless steel implants may also be used for the same indications as an adjunct to fusion.
With the exception of DDD, CD Horizon™ Legacy™ 3.5mm rods and associated components may be used for indications in skeletally mature patients as an adjunct to fusion. The 3.5mm rods may be used for the specific pediatric indications noted. When used for posterior non-cervical pedicle screw fixation in pediatric patients, CD Horizon™ Spinal System titanium, cobalt chrome, and stainless steel implants are indicated as an adjunct to fusion to treat progressive spinal deformities (i.e. scoliosis, kyphosis, or lordosis) including idiopathic scoliosis, neuromuscular scoliosis, and congenital scoliosis. Additionally, the CD Horizon™ Spinal System is intended to treat pediatric patients diagnosed with the following conditions: spondylolisthesis/ spondylolysis, fracture caused by tumor and/or trauma, pseudarthrosis, and/or failed previous fusion. These devices are to be used with autograft and/or allograft. Pediatric pedicle screw fixation is limited to a posterior approach.
The CD Horizon™ PEEK rods are intended to provide posterior supplemental fixation when used with an interbody fusion cage for patients diagnosed with DDD. These DDD patients may also have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level. This device is intended for 1-2 level use in the lumbosacral spine (L2 – S1) in skeletally mature patients. Devices are intended for use with an interbody fusion cage at the instrumented level and is not intended for stand-alone use.
The CD Horizon™ Spire™ plate is a posterior, single-level, non-pedicle supplemental fixation device intended for use in the non-cervical spine (T1-S1) as an adjunct to fusion in skeletally mature patients. It is intended for plate fixation/attachment to spinous processes for the purpose of achieving supplemental fixation in the following conditions: DDD, spondylolisthesis, trauma, and/or tumor.
To achieve additional levels of fixation, CD Horizon™ Spinal System rods may be connected to the Vertex™ Reconstruction System with the Vertex™ rod connector. Refer to the Vertex™ Reconstruction System package insert for a list of Vertex™ indications.
Anteralign™ Spinal System with Titan nanoLOCK™ Surface Technology System
Anteralign Spinal System with Titan nanoLOCK Surface Technology System interbody cages with macro-, micro-, and nano-roughened surface textured features are intended to be used in spinal fusion procedures on skeletally mature patients with symptomatic Degenerative Disc Disease (DDD, defined as discogenic pain with degeneration of the disc confirmed by patient history and radiographic studies), degenerative spondylolisthesis, and/or spinal stenosis, at one or two contiguous levels from L2 to S1 whose condition requires the use of interbody fusion. These patients may also have up to Grade 1 Spondylolisthesis or retrolisthesis at the involved levels. These patients should have had six months of nonoperative treatment prior to treatment with this device.
Additionally, the Anteralign Spinal System with Titan nanoLOCK Surface Technology can be used as an adjunct to fusion in patients diagnosed with multilevel degenerative scoliosis and sagittal deformity.
The Anteralign Spinal System with Titan nanoLOCK Surface Technology is intended to be used with autograft and/or allogenic bone graft comprised of cancellous and/or corticocancellous bone graft, and/or demineralized allograft bone with bone marrow aspirate or a combination thereof. These implants may be implanted via a minimally invasive OLIF or minimally invasive or open DLIF approach. The Anteralign Spinal System must be used with a posterior supplemental internal spinal fixation cleared for use in the lumbar spine.
Miniplate and bone screw components are provided as an option for anti-migration for the lumbosacral levels oblique or lateral above the bifurcation (L2-L5) of the vascular structures. Indications and contraindications of spinal instrumentation systems should be understood by the surgeon.
O-armTM Surgical Imaging System-Indications
The O-arm™ O2 Imaging System is a mobile x-ray system designed for 2D fluoroscopic and 3D imaging for adult and pediatric patients weighing 60 lbs or greater and having an abdominal thickness greater than 16cm, and is intended to be used where a physician benefits from 2D and 3D information of anatomic structures and objects with high x-ray attenuation such as bony anatomy and metallic objects. The O-arm™ O2 Imaging System is compatible with certain image guided surgery systems.
UC202312610EN CST
©2023 Medtronic. All rights reserved. Medtronic, Medtronic logo and Further, Together are trademarks of Medtronic. All other brands are trademarks of a Medtronic company.